Community call: Managing Health and Technology Research Data

On Tuesday 26th October we hosted a community call on ‘Managing Health and Technology Research Data‘.
The one-hour long event brought participants together from various institutions around the world for a discussion about medical device regulation and publishing medical data.
Download the slides and watch the recording!
Getting to know participants
University of Technology Biomedical Engineering data steward, Anne Aarts, kicked off the first session with a Menti discussion to get to know our community participants.
The majority of participants were research data management support staff who advise researchers about the appropriate handling of medical data.
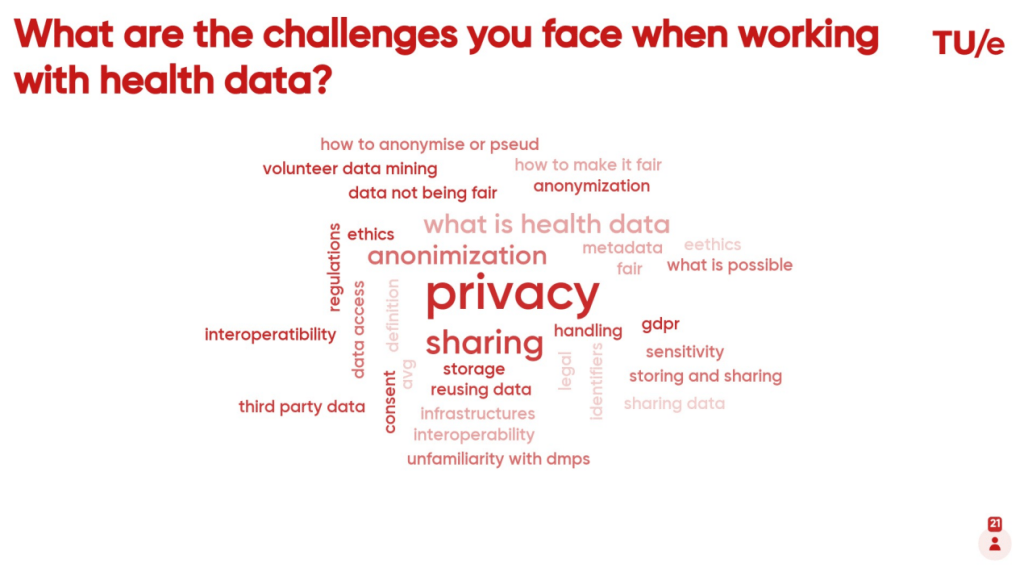
Interestingly, some of the participants revealed that they were not sure whether they work with medical data or not, given the broad definition of ‘health data’ and uncertainty about what ‘health data‘ is.
Other challenges to working with health data were reported to relate to understanding privacy regulations; how to appropriately store, share and reuse health data; how to make health data FAIR; and, how to correctly anonymise/pseudonymise health data.
Session 1: Medical Device Regulation (Anne Aarts)
Anne Aarts was part of the LCRDM Task Group on Medical Device Regulation (MDR). In the first session, Anne introduced the European Regulation that was introduced in May 2021.
The MDR defines a ‘medical device’ as any instrument, apparatus, appliance, software, implant, reagent, material or other article… to be used for human beings for medical purposes.
The task group brought various stakeholders together to create an overview of different existing resources that can be used to support researchers using medical devices throughout various stages of the research lifecycle.
The task group created a poster with ‘take home messages’ that guide researchers through the process of MDR. The poster presents information about:
- Preparing to use a medical device (including information about the type of medical device, it’s characteristics and class; how it will be used in clinical study; and, it’s development and use in-house or externally).
- Roles and responsibilities (e.g. determining who is responsible for the medical device within collaborative projects).
- Research data management and documentation required.
- The registration and approval process involving the research from medical/ethical committee.
The poster will be published by LCRDM soon.
Session 2: Publishing medical data (Kostas Nizamis)
Assistant Professor in Multidisciplinary Design at the University of Twente, Kostas Nizamis, shared his personal experience of managing health data during his doctoral research.
Kostas developed a hand robotic exoskeleton for patients with Duchenne Muscular Dystrophy to assist them with daily activities. This human-machine interface, ‘SymbiHand’, helps the patient to regain some strength in their hand to lift and move objects.
After sharing some background to his research, Kostas talked about publishing his dataset in 4TU.ResearchData.
He believes that:
Sharing data improves the quality of work, makes it FAIR and transparent, and can result in collaborations. High quality work should not fear exposure! By sharing data, others can reuse it without duplicating efforts and recruiting more human participants. This reduce the time, effort and resources invested by researchers and participants.
Reflections on the session:
A few participants provided the following feedback about the call:
- The event was rated very good/excellent.
- They enjoyed the speakers’ presentations, content of the call and interactive Q&A sessions.
- The call could have been longer in duration and calls could be held more frequently throughout the year.
- Follow up discussions are required on medical device regulation and publishing medical health data.
- It would be nice to learn about tips, tricks and pitfalls of sharing data and how to plan to share data from the beginning of a research project.
Thank you to the speakers and participants for an interactive and interesting discussion. We look forward to the next community call.
If you have ideas for topics and would like to help us organise our next community call, please contact us!
We’d love to hear from you 🙂